This post deviates a from my normal blog topic (making alcoholic beverages). Why is prohibition important? Well, Alberta still has legislative and regulatory "quirks" that are holdovers from the days of prohibition in Alberta. For example, in home winemaking, there is no legal way for a wine kit supply store in Alberta to provide customers with a clean, physical space inside the store in which to make wine, store it as it ferments, and bottle the wine when it's ready. These services are popular in other provinces, but are not allowed in Alberta, because such a service goes beyond "home winemaking". Governments in Alberta are liberalizing the liquor laws, but there is a long way to go. An interesting documentary account of the history of beer in Alberta, including the impact of prohibition, can be found at:
Aleberta
Some time ago, I was surfing through the photo collection of the
Provincial Archives of Alberta, and came across a bunch of old "Temperance posters". These posters were published by "The Dominion Scientific Temperance Committee", which, I gather, was an arm of the
Women's Christian Temperance Union back in the days of prohibition. Some of these posters are quite amusing:
 |
| Provincial Archives of Alberta, Object number PR1974.0001.0400a.0001 |
 |
| Provincial Archives of Alberta, Object number PR1974.0001.0400a.0006 |
It is not clear to me what phagocytosis (the white blood cell "swallowing and digesting" germs) has to do with alcohol consumption. Clearly, the idea was to scare people away from consuming alcohol, and, I suppose, get them to drink water instead.
The next poster piqued my interest because it touches on some chemistry.
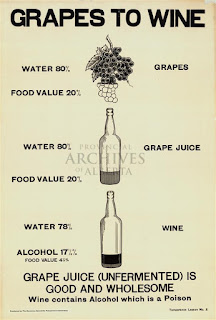 |
| Provincial Archives of Alberta, Object number PR1974.0001.0400a.0002 |
The poster argues that the nutritional value of grape juice is much greater than that of wine, but the numbers don't make a lot of sense. The only number that looks correct is the 20% "food value" of grape juice. That number roughly corresponds to the percentage (by mass) of sugar in grape juice. However, the percentages of water, alcohol, and "food value" in wine do not make sense.
1) The water content of wine is a lot higher than 78%. Water is a by-product of the fermentation of sugar. So, during fermentation, sugar decreases, and the proportion of water and alcohol increase.
2) The poster gives 17.5% alcohol for wine. Is this 17.5% by mass or 17.5% by volume? On wine, beer, and liquor labels, alcohol content is usually listed as a percent by volume. 17.5% would be on the high end of percent volume for wine. This poster, however, seems to be using mass percentages. The mass percentage should be a lot lower, because the density of pure ethanol is less than that of water. So, there is something funny going on.
3) The food value of wine is listed as 4.5%. Sure, wine contains residual sugars and other organic components that have nutritional value, but this number appears to have simply come from subtraction: 100 - (78 + 17.5) = 4.5. But, as the percentages of water and alcohol are suspect, so is the 4.5% value.
4) The poster implies that alcohol has zero food value, which is incorrect! The caloric content of foods and beverages can be determined through calorimetry: In the good old days, a scientist would combust a sample of food in a sealed chamber and measure the amount of heat produced. Calorimetry is a lot of work, so nowadays, the caloric content of foods is determined indirectly using the "Atwater system" (Scientific American has an article on this,
here). Basically, this involves adding up reference caloric values for different components of the food or beverage in question. The reference caloric value of carbohydrates (including sugar) is 4 kcal/g (1 kcal = 1 food Calorie). The reference value for alcohol is... wait for it... 7 kcal/g! (Yes, on the basis of mass, alcohol has more food Calories than sugar.) So, the poster is WRONG.
The web site
Compound Interest has a lot of neat infographics about various familiar substances. Take a look at their infographic on
red wine. Their numbers make a lot more sense (86% water, 12% alcohol, 2% other organic compounds).
By the way, the "food value" of a 5 oz. glass wine is around 125 Calories, which is equivalent to 25 jelly beans. The glass of wine is arguably the healthier option of the two!
These temperance posters were published ca. 1912. Are these scientific mistakes forgivable, given that they were made over 100 years ago? Not really. Even in 1912, physics and chemistry were sufficiently advanced that mass and volume compositions were well understood and measured reliably. And, calorimetry was already an established experimental technique in the field of thermodynamics, which had its heyday in the age of steam engines. What's going on here is the twisting of information to support a particular agenda. It's pseudoscience!












